Vedic has been focused on Nutraceuticals, Dietary supplements and Branded ingredient preclinical and clinical trials for 22+ years.

Human Clinical Trials
Pilot studies, Proof of concept studies and efficacy Human Clinical Trials – from customized study design to manuscript publishing, Vedic houses end-to-end services. Got a regulatory submission like Novel food and GRAS to make? We cover that as well!
- Medical Writing
- Study design
- Protocol development
- Investigator’s brochure
- Clinical Study Report
- Institutional Review Board (Ethics Committee)
- Project Management
- Study Recruitment
- Sponsor communication and reporting
- Investigator’s Meeting
- Quality Assurance
- Site Monitoring
- Site Audits
- Clinical Data Management (Electronic)
- Statistical Analysis (Biostatistics)
- Objective and Subjective Tests
- Objective Measurements (Biomarkers)
- Subjective (Questionnaires, scales and computerised assessments)
- Research publishing
- Journal publishing
- Types of Clinical Trials
- Proof of Concept / Pilot Studies
- Bioavailability / Bioequivalence Trials
- First-In-Human Studies
- Large sample size Human clinical Trials

Preclinical Efficacy
Customized rodent models available on and above the available preclinical efficacy study designs for health claims like metabolism, sleep, neurobehavioural, AMPK, anti-diabetes, weight management and more!
- In-vivo models
- Neurobehavioural Activity in Rodent
- Anti-Inflammatory & Antioxidant Activity in Rodent Model
- Exercise & Endurance performance in C57BL6 Mice
- AMPK Activation in Exercising Mice
- Assessment of Enzyme Systems Downstream upon AMPK Activation in Exercising Mice Model.
- Liver Disease - NASH Model in Mice
- Liver Disease - Paracetamol-induced acute hepatotoxicity model in mice
- Assessment of Aging Markers In Rat Model
- Preclinical Efficacy Study to Examine the Effect of the Test Item (Designed for men’s health) in a Rat model
- All animal models are supported by histopathology, biomarker analysis, and relevant mechanistic assays.
- Metabolic disorders: diabetes (rats), obesity (mice), hyperlipidaemia (rabbits and hamsters), dyslipidaemia (Guinea Pig), atherosclerosis (rabbits)
- Inflammation & hypersensitivity: Pulmonary Inflammation, Atopic dermatitis, Psoriasis, Arthritis
- Peptic Ulcer models
- Hepatoprotective activity in mice
- Allergic conjunctivitis
- Charcoal meal test in rats and mice
- Forced Swim and Tail suspension test in mice
- Longevity Study in Nude Mice (6-12 months study)

Toxicity Studies
Vedic follows global standard OECD guidelines and FDA Red book guidelines available for conducting Toxicity studies. Do you know OECD 443?!
- Acute oral toxicity study (OECD 425)
- 14 day study
- 28 day study (OECD 407)
- 90 day study (OECD 408)
- In-vitro Chromosomal Aberration Test (OECD 473)
- In vitro Mammalian Cell Gene Mutation Assay on L5178Y Mouse Lymphoma cells TK+/- with test item (OECD 490)
- Mammalian erythrocyte micronucleus test of test item in Swiss albino mice (OECD 474)
- Bacterial Reverse Mutation Assay of test item Using Salmonella typhimurium and E. coli Tester Strains (OECD 471) also known as AMES test
- In-vitro Mammalian Cell Micronucleus Test Using Chinese Hamster Cell Line Culture (OECD 487)

Types of products
- Dietary supplements
- FMCG
- Medical devices
- Medical foods
- Traditional medicine
- Direct to consumer products making health claims
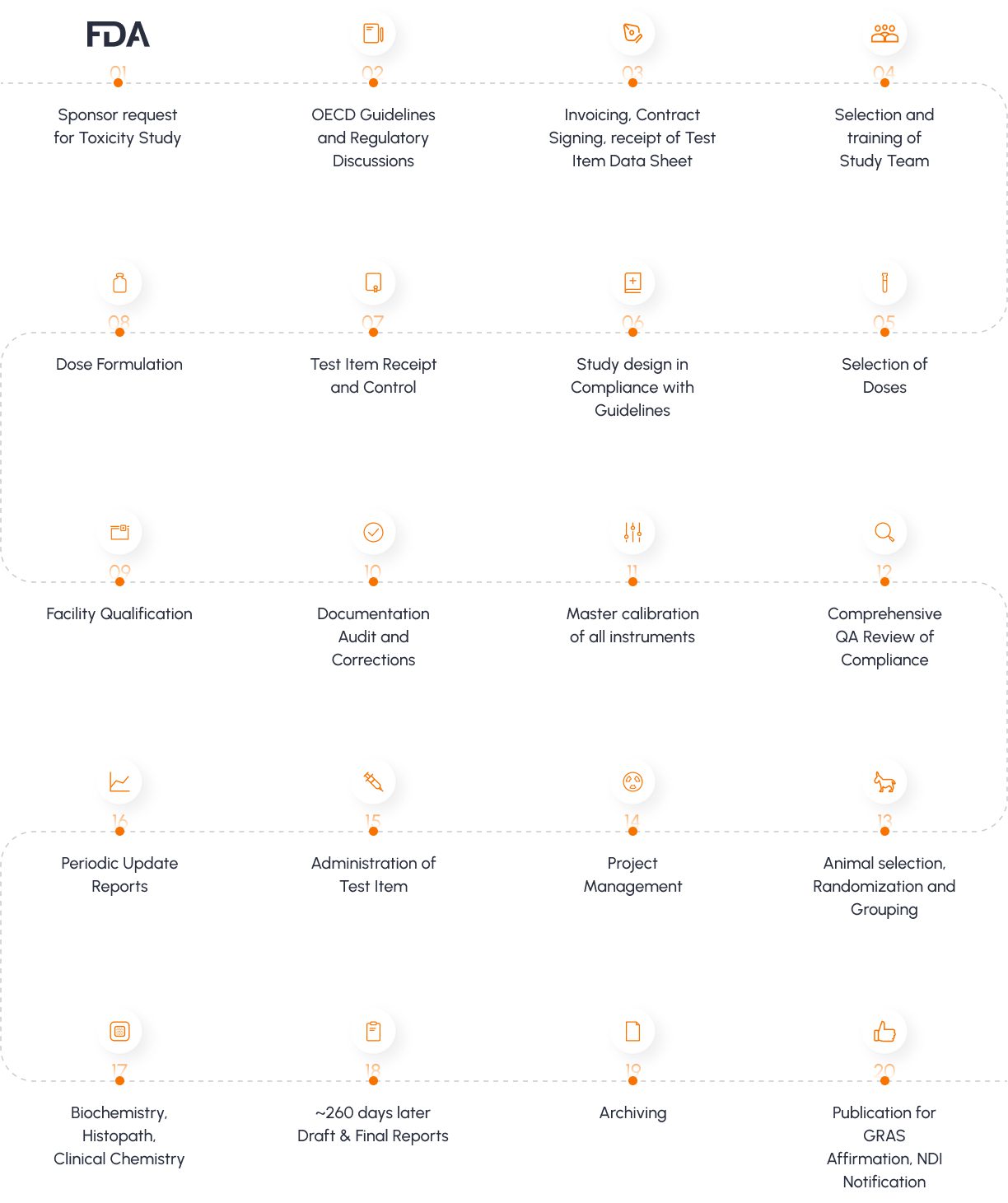
The Vedic Process
Quick glimpse at Vedic’s standard operating procedure for conducting Preclinical safety, efficacy and Toxicity studies
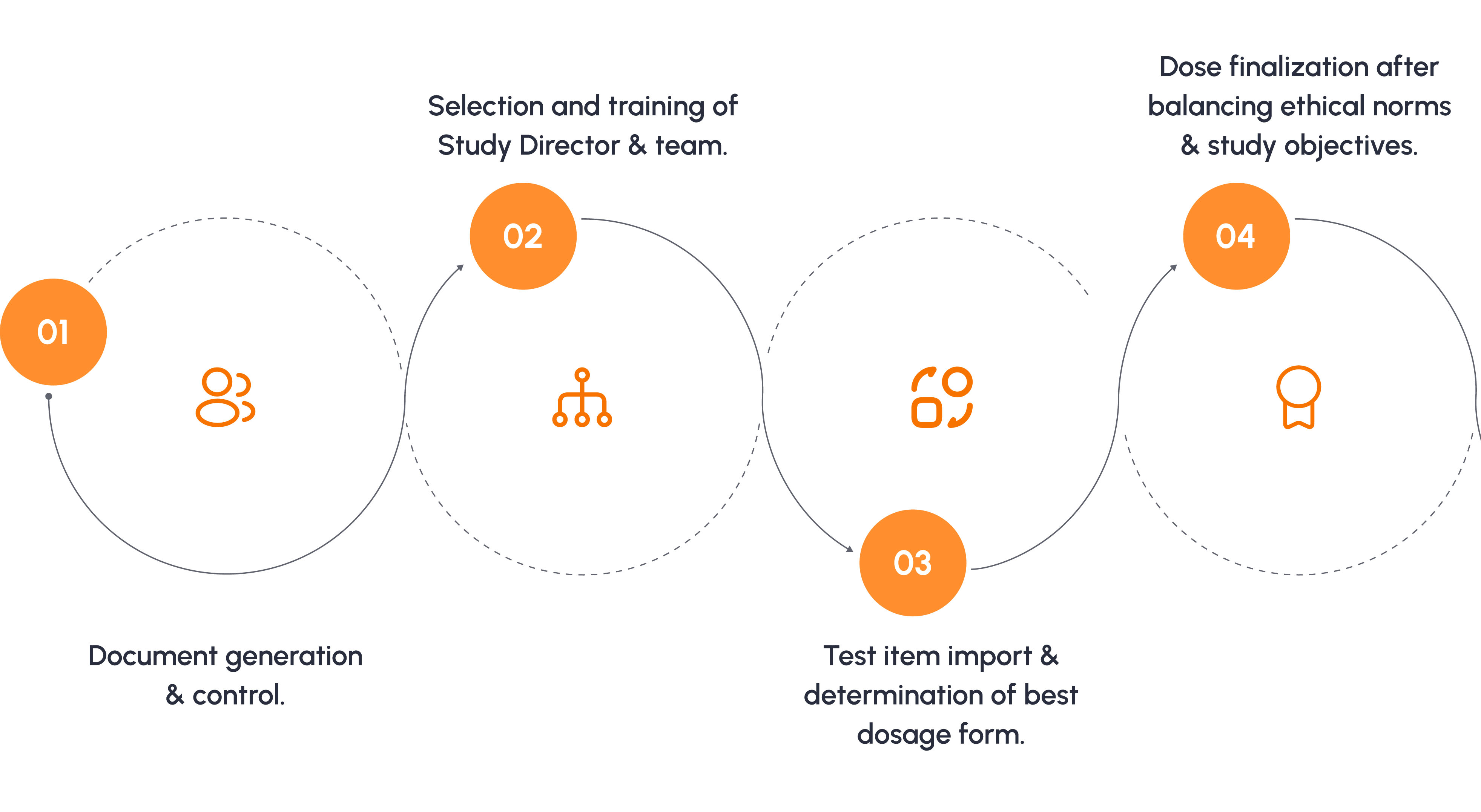
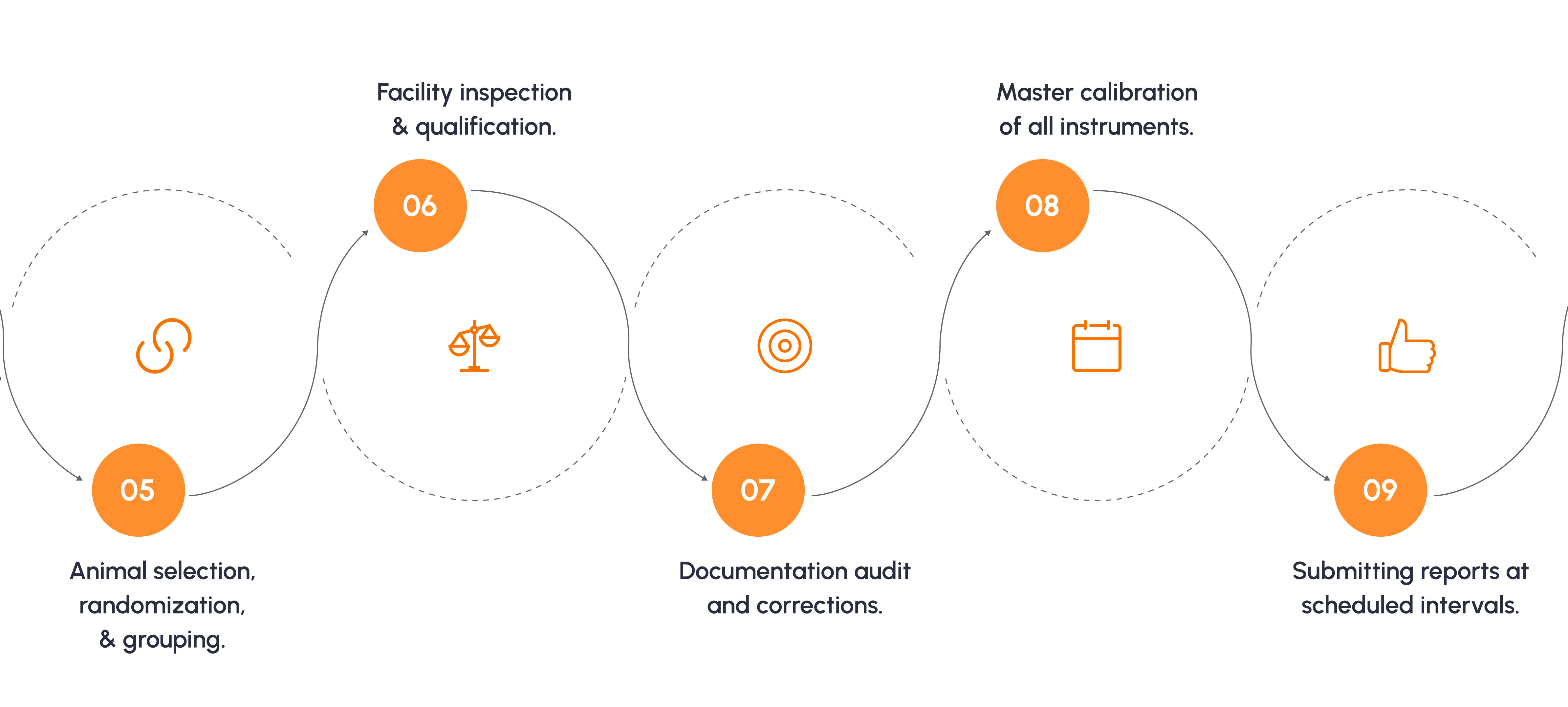
Typical Toxicology Study Process
For a 90 day oral toxicity study as per OECD 408.