
Probiotic clinical trials: What are probiotic researchers studying? How are studies being designed?
A report on the current global landscape of probiotic clinical trials.
The symbiotic relationship between the commensal microbiota and the human host is the subject of extensive ongoing research. A wide spectrum of researched disciplines—ranging from gastroenterology, immunology, and microbiology to nutrition, food science, and many more—are increasingly interested in investigating the functional, nutritional, and therapeutic potential of probiotics for human health. The underlying rationale for the research is the potential role of probiotics in enhancing the host’s internal homeostasis, thereby promoting human health.
This report provides an understanding of the current global landscape of clinical trials investigating the potential of probiotics to promote human health. Our company, contract research organization (CRO) Vedic Lifesciences, compiled an extensive database of the clinical trials conducted on probiotics spanning the last five years.
Our medical team analyzed approximately 400 studies to provide an in-depth assessment of the current trends in probiotic studies, including the countries leading the research in this area, the healthcare disciplines in which probiotics are most investigated, industrial engagement in the concerned clinical trials, and the preferred study population for these studies, alongside additional parameters. This is probably the most comprehensive report that exists on this subject.

Scope, Terminology, and Methods
Scope
This report covers human intervention studies using the keyword probiotic in their study titles. It notes the trends seen in probiotic clinical trials, yielding insights on parameters to consider while designing a clinical study pertaining to such products/health areas. The whole idea is to help dietary supplement companies visualize the kind of claims they would like to associate with their next probiotic ingredient or finished product, which tools could be helpful in substantiating those claims, and how they should go about planning such a project; in short, this report is a good starting point for some “secondary research” before designing your next probiotic clinical study. It also provides some market understanding for new product development teams.
Terminology
Clinical trial: A study in which an investigational product is administered to a group of volunteers (often in comparison to a placebo) to check the efficacy, safety, and/or tolerability of the product in a particular health area based on the product’s mechanism of action. The study often also measures the onset of action, the extent of effect, and the type of effect using objective and subjective tools.
Clinical trial registry: An online platform on which a clinical trial is “registered” before the commencement of the study. Details entered include, among other information, the study’s title, estimated start and completion dates, and the study design, objectives, tools, etc. Most countries maintain their own clinical trial registries as a necessity given that each country follows different regulations.
Methods
Source: This report’s information is based on probiotic clinical trial data sourced from www.clinicaltrials.gov. This U.S.-based registry is one of the most popular portals for universities and companies by which to register their studies conducted all over the world. For this report, we chose to use the clinical trial registry www.clinicaltrials.gov instead of a search engine like PubMed, which would only have yielded trends from studies initiated two or more years ago, whereas a clinical trial registry provides more current data, including data on studies not yet completed.
Keyword: We searched for the keyword probiotic in the “Study Title” field using Advanced Search filters; hence, a study that does not contain this keyword in its title will not be covered in this report.
Filters: Date filter: Study started January 1, 2015–November 30, 2019
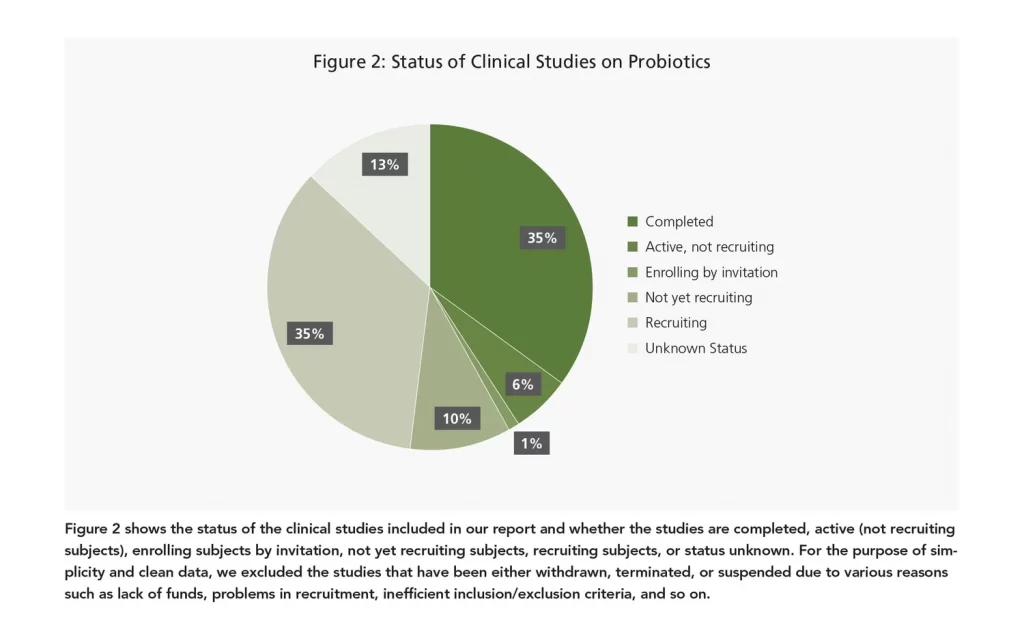
Status filter:
- Not yet recruiting
- Recruiting
- Enrolling by invitation
- Active, not recruiting
- Unknown status
Fast Facts from This Report
- 392 = Total number of studies that were registered in the period of January 1, 2015–November 30, 2019, containing the keyword probiotic in the study title
- 44,360 = The total number of volunteers cumulatively covered in the studies in this report
Probiotics have shown promise for a variety of health purposes, including prevention of antibiotic-associated diarrhea (including diarrhea caused by Clostridium difficile), prevention of necrotizing enterocolitis and sepsis in premature infants, treatment of infant colic, treatment of periodontal disease, and induction or maintenance of remission in ulcerative colitis.
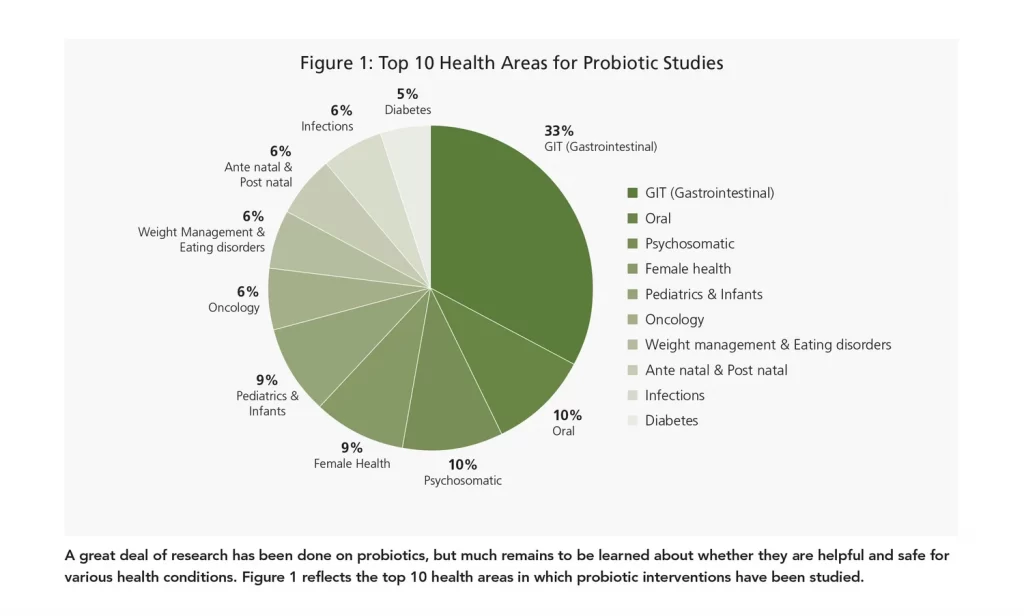
Naturally, the top trending area of research is gastrointestinal (GIT), which spans such conditions as short bowel syndrome, ulcerative colitis, excessive gas accumulation, eradication of Helicobacter pylori, antibiotic-associated diarrhea, and constipation symptoms. Research has been performed in such populations as older adults with dysphagia, seniors with increased intestinal permeability, university students experiencing stress-associated GIT conditions, etc.
The probiotic studies done on oral health mainly span such conditions as periodontitis, dental caries, gingivitis, postoperative complications, halitosis, peri-implantitis, and malocclusion.
Another common probiotic research area is brain health, including stress, mood, anxiety, depression, dementia, obsessive-compulsive disorder (OCD), mental fatigue, attention deficit hyperactivity disorder (ADHD), and Parkinson’s disease. Infant health is another focus area, including neonatal hyperbilirubinemia (jaundice), otitis media with effusion (OME), acute diarrhea, stunting, respiratory infection (such as pneumonia and bronchitis), severe acute malnutrition, and infantile colic.
Still-developing areas of research for probiotics include weight management and eating disorders, metabolic syndrome, and immunity.
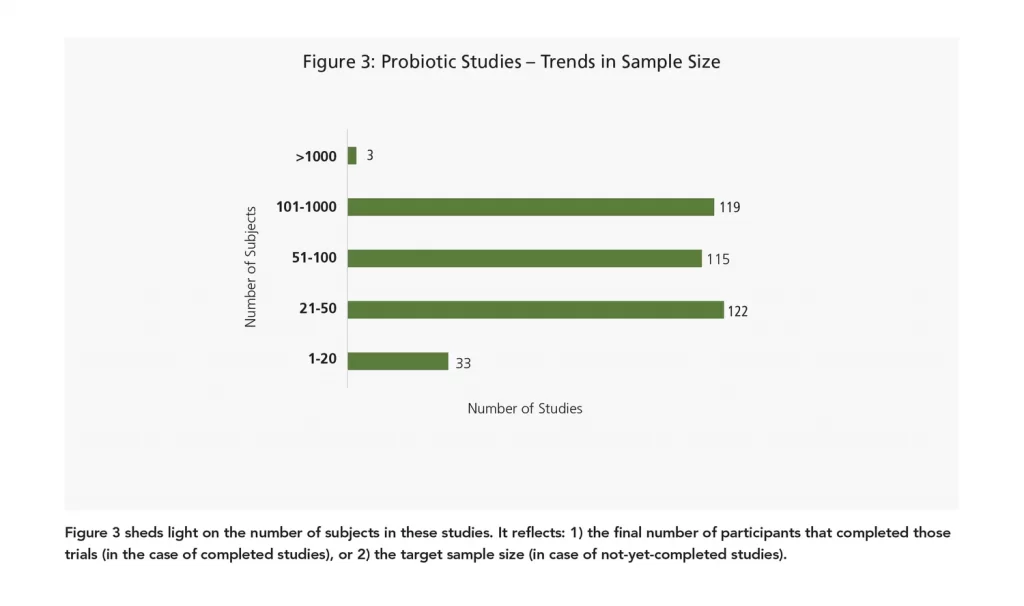
Let’s take a look at some of the smaller probiotic studies (studies with a sample size of 1-20). Also known as proof-of-concept trials, these smaller studies are being done in various health areas, ranging from probiotics used to “calm” your mind to probiotic supplementation in iron-deficient pregnant women. Interestingly, a healthcare organization based in New York is currently enrolling for a prospective trial to see the effects of a probiotic formulation on inflammatory and stool profiles in patients with non-alcoholic fatty liver disease (NAFLD). Serum cytokine levels, including IL-10 and IL-17, will be assessed.
As opposed to the niche study targets of smaller probiotic trials, the largest probiotic studies focus on tried-and-tested zones like osteoarthritis, blood pressure management, and urinary tract infection (UTI). Overall it is recommended to start exploring a new concept with a pilot study and to follow up with a larger study once the concept/effect has been established in a particular study population. The larger study may involve more secondary objectives, tests, tools, or biomarkers.
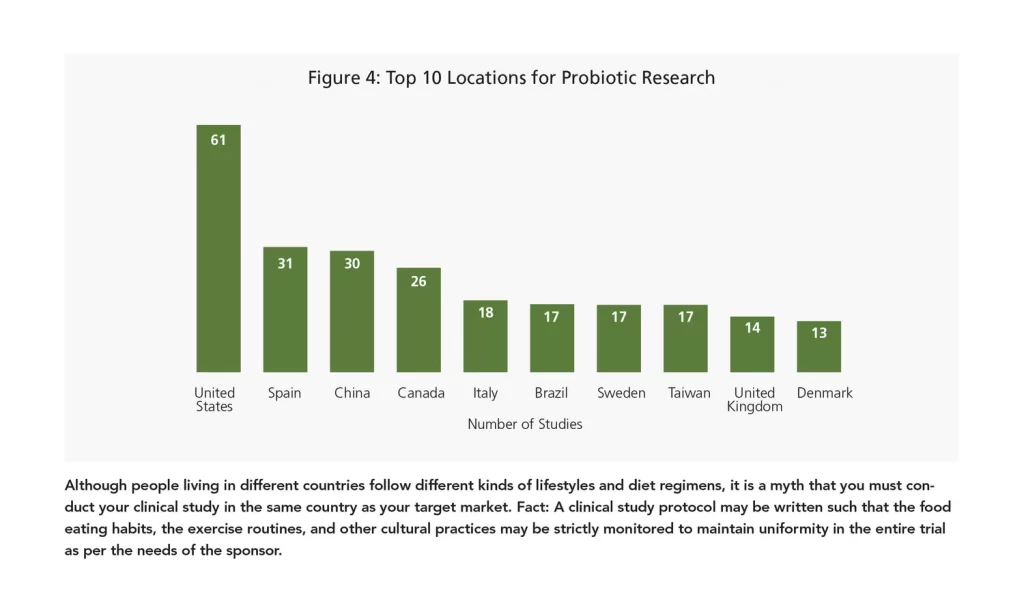
The United States of America, the dietary supplement hub of the world, has access to state-of-the-art clinics, hospitals, and pathology labs, thus serving as one of the top study sites for probiotics; however, a lot of high-quality research is currently taking place by researchers all over the world, including Europe, Asia, and South America. China is one of the key destinations in which to conduct studies due to the country’s sheer population size and affordable prices of conducting research. Countries like India and Malaysia may emerge as leading locales for probiotic studies as well.
Another note: Marketing teams often push clinical trial managers to get studies done faster so the data can be used for promotional purposes. This is where fast recruitment, disciplined project management, and on-time report delivery comes in. To this end, every country offers its own advantages and disadvantages. The ideal locale depends on the study requirements, the budgetary constraints, and the geographical needs of the sponsor. For example: A study testing the effects of a skin health supplement on skin elasticity, moisture, sagginess, etc., when skin is exposed to air pollution may be best done in a place like Mumbai, India, which has an Air Quality Index of 635, which is considered hazardous.
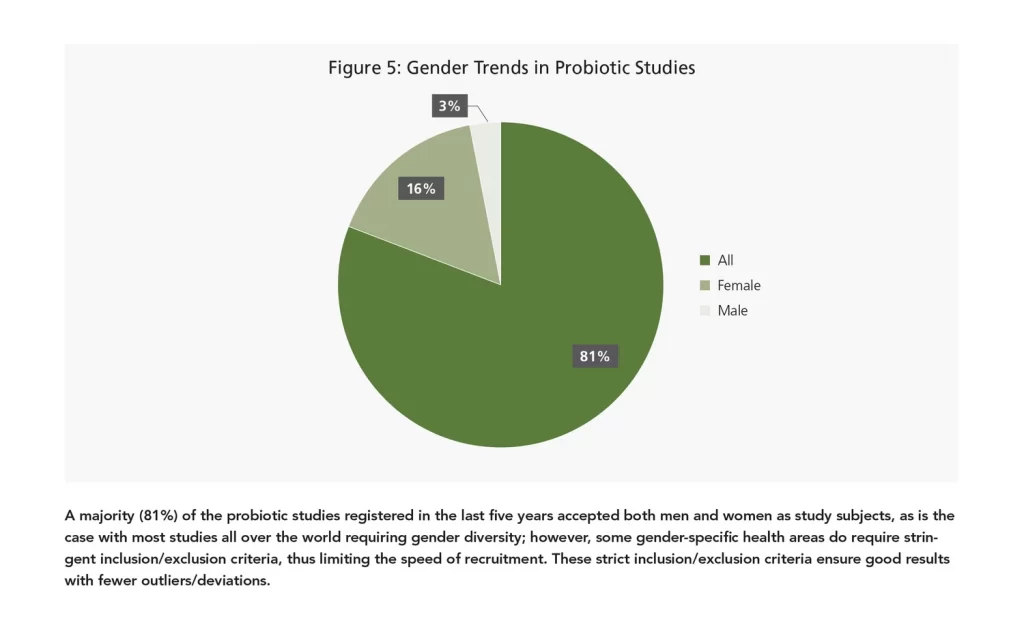
Women-centric trials include studies testing probiotic strains in areas such as UTIs, vaginal candidiasis, polycystic ovary syndrome (PCOS), pregnancy-related conditions, menopausal symptoms, and bone health. Very few probiotic studies focus on male volunteers exclusively, and those that do have primarily focused on examining markers of muscle damage and performance following exercise-induced muscle damage. Females are generally excluded from these studies to avoid huge deviations in the data. Also, females will respond differently than males in exercise/fitness-related studies. Again, choosing the right gender mix for your next probiotic study really depends on your target claim, desired effect, and type of audience you wish to target.
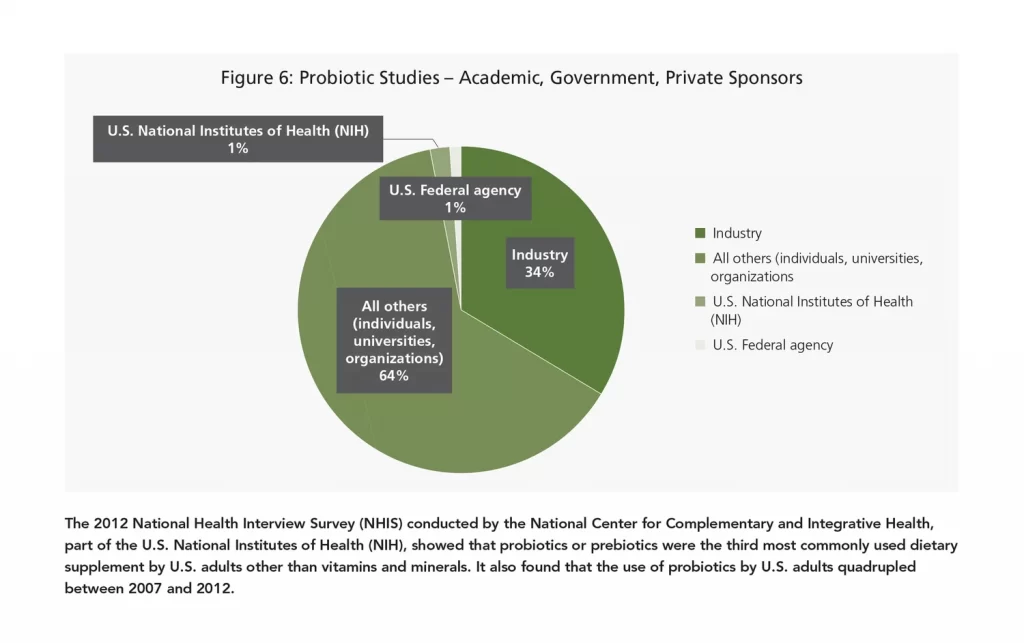
Rising probiotic consumption is due in large part to expansion of probiotic products into new sectors, intensive R&D on probiotic products, and product innovations, all of which are orchestrated by the top market vendors. More academia and dietary supplement companies are now taking the plunge into this ever-growing sector. Over the last five years, leading players such as Bifodan, BioGaia, Chr. Hansen, Danone, Lallemand, Nestlé, and Probi have invested in clinical research to substantiate their product claims. This heavy investment by healthcare experts all over the world indicates the immense potential of this industry and the countless untapped market possibilities.

Shorter probiotic studies include trials like those assessing the tolerability of a probiotic dietary supplementation in healthy adults (10 days) or examining the effects of a one-month probiotic treatment on mental fatigue. Mid-range interventional periods are often selected for studies such as those on osteoarthritis (6 weeks), hypertension (8 weeks), periodontitis (12 weeks), effects on withdrawal symptoms after smoking cessation (14 weeks), and symptoms of depression (16 weeks).
Longer studies are generally required for more serious conditions where probiotics take longer to show effect, such as advanced chronic kidney disease (20 weeks), ADHD (24 weeks), gestational obesity (28 weeks), infertility (30 weeks), weight loss maintenance (36 weeks), prevention of necrotizing enterocolitis (40 weeks). The studies that typically last for a year or longer address conditions such as diabesity, eradication of Helicobacter pylori, preventing preterm birth with probiotics, respiratory tract infections in children, management of IBS-D (irritable bowel syndrome that causes increased diarrhea), and bone mineral density in healthy women in the early postmenopausal phase.

The amount of time a study takes to complete depends on the following factors:
- On-time availability of the clinical trial supplies (investigational product and placebo)
- The past experience, expertise, and skills of the organization conducting the study (e.g., a university or CRO)
- The time taken to select the study sites and prepare all the documents required for institutional review board (IRB) review
- The time taken for IRB approvals: Some countries have faster processes than others. Also, hospital IRBs meet less frequently than IRBs not associated with hospitals or nursing homes.
- Time taken for recruitment: This is the key aspect that plays a very important role in affecting the speed of the study. The recruitment speed depends on the access to recruiters (particularly important in the case of healthy-volunteer studies) and investigators (doctors) with good patient pools (in the case of diseased-population or post-market-surveillance studies), as well as the efficiency of site coordinators.
- Time taken for data analysis and report writing: An experienced CRO will definitely have high-quality electronic data-capture systems that will help speed up the process, reduce data entry errors, and audit findings. It will also have commendable medical writing teams who can write CONSORT-friendly clinical study reports and manuscripts favored by high-impact-factor scientific journals targeted for publication.
About the Author
Kriti Chaudhary heads business development and strategy for Vedic Lifesciences (Mumbai, India), one of the most experienced contract research organizations (CROs) serving the dietary supplement industry. A pharmacist-MBA by education, Chaudhary has a keen interest in nutraceutical product development, clinical research trends, and functional food innovation. Vedic Lifesciences not only helps ingredient and finished-product companies with clinical trials and animal studies but also calls itself the “Claims Guru”—an advisor of claims and how to substantiate them. In the past two decades, Vedic has conducted and published work in health areas such as joint health, cognition, gut health, sports performance, heart health, and many others. Visit https://vediclifesciences.com and connect@vediclifesciences.com.


